

Look at Least-Common-Multiple.html if you're not sure about how the subscripts in iron(III) sulfide came to be.Įxample #4 - Write the formula for: tin(IV) phosphide Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom. Image credit: Wikipedia Commons, public domain. Step #4 - since a formula must have zero total charge, you write the formula Fe 2S 3. Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Step #3 - Sulfide (the anion) means S 2¯. remember, that comes from the Roman numeral. Step #2 - the charge on the cation is a positive three. Step #1 - the symbol of the cation is Fe. Step #4 - since a formula must have zero total charge, you write the formula Cu 2O.Įxample #3 - Write the formula for: iron(III) sulfide Step #3 - the anion symbol and charge comes from the second name. Step #2 - the Roman numeral WILL tell you the charge on the cation. Step #1 - the first word tells you the symbol of the cation. Chloride is the name and Cl¯ is the face.Įxample #2 - Write the formula for: copper(I) oxide The ChemTeam is often asked by students, "But how do you know that chloride means Cl¯?" That type of question is usually answered with a question, as in "How do you know the name and face of your best friend?" That's right, you've spent time in their company, to the point where you have memorized the connection between name and face. On writing the formula from the ions, you may want to review Charge-Crossing.html or Least-Common-Multiple.html for more information. Step #4 - remembering the rule that a formula must have zero total charge, you write the formula CuCl 2. This suggestion has not been followed, but the Stock system remains in use world-wide.Įxample #1 - Write the formula for: copper(II) chloride In 1934, Stock approved of the Roman numerals, but felt it better to keep the hyphen and drop the parenthesis. For example, FeCl 2,which would have been named iron(2)-chloride according to Stock's original idea, became iron(II) chloride in the revised proposal. In 1924, a German commission recommended Stock's system be adopted with some changes. In his own words, he considered the system to be "simple, clear, immediately intelligible, capable of the most general application." It was designed by Alfred Stock (1876-1946), a German chemist and first published in 1919. The type of naming you will learn about is called the Stock system or Stock's system. The ChemTeam holds their students responsible for: Cu, Fe, Hg, Pb, Sn, Mn, Co, Au, and Cr. Your teacher will hold you responsible for the cations you must learn. The anions involved have only one charge. The cations involved in this lesson have AT LEAST TWO charges. The four formulas above are all examples of this type.
IRON III SULFIDE CHARGE HOW TO
This lesson shows you how to write the formula of a binary compound from the word name when a cation of variable charge is involved. There can also be several of each element such as Fe 2O 3 or SnBr 4.
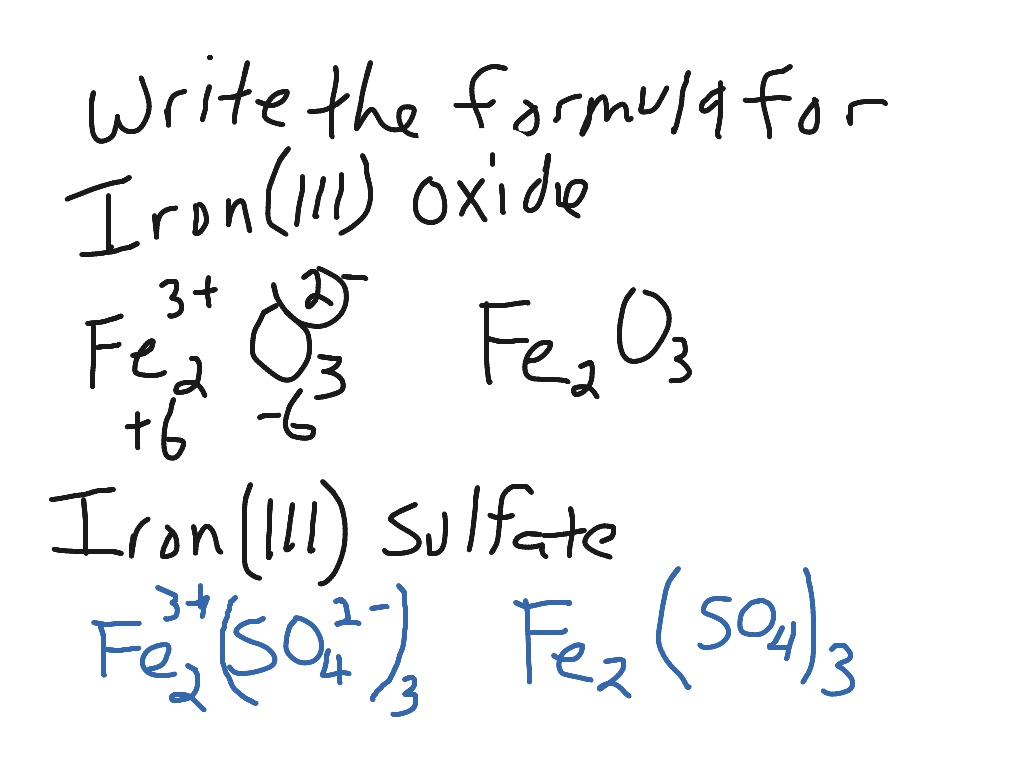
There can be one of each element such as in CuCl or FeO. P ( Weak national response ) = 0.30 P (Moderate national response ) = 0.60 P ( Strong national response ) = 0.ChemTeam: Nomenclature-Binary Compounds:Stockīinary Compounds of Cations with Variable ChargesĪ binary compound is one made of two different elements. Some metal cations always have the same charge, for example, N a X +, M g X 2 +, A l X 3 +, \ce)=0.10 There are two types of catiosn that we may encounter. To name ionic compounds we should always name the cation first and then name the anion. (a) the reaction of sodium hydroxide and iron(II) chloride to give iron(II). Let's recall some simple rules applied when naming ionic compounds. In the above equation, the overall charge is zero, or neutral, on both sides. In this task, we are given an ionic compound and we have to determine its formula.


 0 kommentar(er)
0 kommentar(er)
